Explain the Major Differences Between Covalent and Ionic Bonding
This means that both the atoms are exerting an attractive. There are two types of chemical bonding covalent and ionic.

Difference Between Ionic And Covalent Bonds Compare The Difference Between Similar Terms
Here participating atoms have a high electronegativity difference.
. Covalent--there is electron shari. Covalent - A shared pair of electrons resulting in both atoms having full outer shells. Ionic--there is electrostatic attraction between oppositely charged ions.
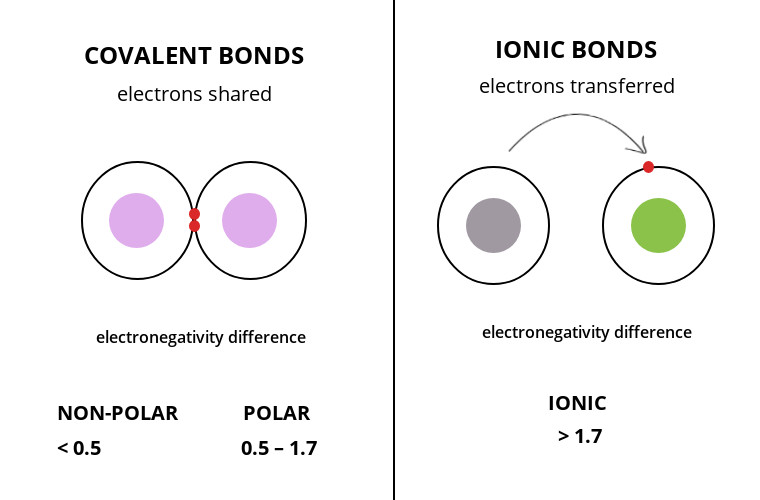
The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. Covalent bond occurs when atoms share their outer shell electrons with each other while ionic bond occurs when one atom donates an electron to another atom. Covalent bonds involve sharing of electrons in the valence shell metallic bonds are the attraction between the delocalized electrons present in the lattice of the metals and ionic bonds are referred as the transferring and.
222aThe main differences between the various forms of primary bonding are. Since electrons have a negative charge this leaves the donating atom electron deficient ve charged ion and the other atom electron rich -ve. In covalent bonding electrons are shared between two atoms.
The main reason for these differences is the difference in their bonding pattern. Formed by a sharing of electrons between two atoms. Formed by transfer of an electron from one atom to another.
Here participating atoms have almost the same electronegativity. Explain the difference between ionic and covalent bonds. Covalent and ionic compounds can be differentiated easily because of their different physical properties based on the nature of their bonding.
Ionic bonding occurs when transfer of electrons takes place. Covalent bond have low polarity while ionic bond has a high polarity. Atoms with partially full outermost electron shells sharing of electrons generally stronger than ionic bonds.
About Covalent and Ionic Bonds. Each atom consists of protons neutrons and electrons. 7 rows Ionic and covalent bonds are fundamentally different in the way they are formed.
A Briefly cite the main differences among the ionic covalent and metallic bonding. Core Difference Between Covalent and Ionic Bonds. Ionic bond involves complete loss or gain of pair of electrons between two atoms whereas in covalent bond only sharing of electrons takes place.
This definition also holds true but again we can now see by use of the electron configurations why these bonds form the way they do. Bonds in Covalent molecules are made using the Valence Electrons. Ionic bonding is between a metal and a non-metal whereas covalent bonding is between 2 non-metals.
9 rows Difference Between Ionic Covalent and Metallic bonds - The major difference between Ionic. Ionic bond has no definite shape while covalent bond has a definite shape. A compound has the high melting point.
So those electrons belong to both of those atoms. The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the sharing is so unequal that an electron from atom A is completely lost to atom B resulting in a pair of ions. One atom or molecule donates one or more electrons to another.
Here are some differences. If you look at an actual image of say the covalent bond between two Hydrogen atoms you can see that the electron cloud surrounding these two atoms sort of merge between them. The main difference is that covalent bonding is the sharing of electrons whereas in ionic bonding electrons are transferred.
7 rows An ionic bond essentially donates an electron to the other atom participating in the bond. -In an ionic bond the atoms are bound together by the attraction between oppositely-charged ions-In a covalent bond the atoms are bound by shared electrons. Therefore their bonding pattern can be deemed as the key difference between ionic and covalent compounds.
Hydrogen bonding involves bonding of highly electronegative elements like fluorine oxygen and nitrogen with hydrogen. Unlike ionic bonds covalent bonds form from the sharing of electrons. Difference Between Ionic and Covalent bond Ionic vs Covalent bond In chemistry a molecule and compound is formed when two or more atoms connect to each other via a chemical process known as bonding.
In the ionic form of chemical bonding the atoms that are linked together do so by. What is the difference between ionic covalent and metallic bonding. We defined covalent molecules as being made of non-metals.
Difference Between Ionic and Covalent Bonds When ionic bonds are formed electrons is donated by a metal and donated electrons is accepted by a non-metal. Ionic is losing and gaining electrons Covalent is sharing the same electrons Ionic - One atom loses and electron the other gains one and two oppositely charged ions are produced which are attracted to each other. The compound has the low melting point.
Learn vocabulary terms and more with flashcards games and other study tools. In ionic bonding the metal loses the electrons in the outer shell to become a positive ion with a full outer shell and the non-metal gains these electrons to. Oppositely charged ions that are attracted to each other are bound into molecules by ionic bonds giving and taking electrons Covalent bonds.
Start studying Difference Between Ionic and Covalent Bonds. Covalent bonds occur between two non-metals metallic bonds is between two metals while ionic is observed between non-metal and metal. At room temperature and normal atmospheric pressure covalent compounds may exist as a solid a liquid or a gas whereas ionic compounds exist only as solids.
View the full answer. In general metallic elements tend to form ionic bonds and non-metallic elements tend to form covalent.
Difference Between Covalent And Ionic Bonds
15 Major Difference Between Covalent And Ionic Bonds With Table Core Differences
What S The Difference Between An Ionic Bond And A Covalent Bond Quora
Comments
Post a Comment